Slim 10 saga (2002): Difference between revisions
Dayana Rizal (talk | contribs) No edit summary |
Dayana Rizal (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
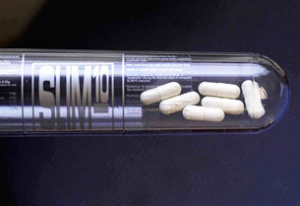
[[File:Slim 10 pills Singapore.jpg|thumb|''The Slim10 pills that had been distributed in Singapore.'' ]] | [[File:Slim 10 pills Singapore.jpg|thumb|''The Slim10 pills that had been distributed in Singapore.'' ]] | ||
Slim 10 slimming pills were first brought into Singapore in November 2001.<ref>“Slimming agent may affect heart”. ''The Straits Times.'' May 1, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020501-1.2.45.20</ref> They were widely popular, with local actress Chen Liping fronting their marketing campaign in December 2001. However, cases of consumers experiencing adverse health side effects came to light in 2002.<ref>Liang, H.T. “13 down from hepatitis, thyroid problem after taking Slim 10”. ''The Straits Times.'' May 7, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020507-1.2.25.13</ref> Selvarani Raja, a Slim 10 user had died from liver failure caused by the pills. Local actress Andrea De | Slim 10 slimming pills were first brought into Singapore in November 2001.<ref>“Slimming agent may affect heart”. ''The Straits Times.'' May 1, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020501-1.2.45.20</ref> They were widely popular, with local actress Chen Liping fronting their marketing campaign in December 2001. However, cases of consumers experiencing adverse health side effects came to light in 2002.<ref>Liang, H.T. “13 down from hepatitis, thyroid problem after taking Slim 10”. ''The Straits Times.'' May 7, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020507-1.2.25.13</ref> Selvarani Raja, a Slim 10 user had died from liver failure caused by the pills. Local actress Andrea De Cruz underwent a liver transplant after her liver had failed as well. Both cases led to lawsuits.<ref>The Must Share News Team. “The Slim 10 Saga: Confusing Verdicts In Singapore Explained — Part 3”. ''MustShareNews.'' August 10, 2017. Accessed on 15 April 2019. Retrieved from: https://mustsharenews.com/slim-10-saga/</ref> | ||
==Details of the incident== | ==Details of the incident== | ||
Latest revision as of 18:35, 6 May 2019
Slim 10 slimming pills were first brought into Singapore in November 2001.[1] They were widely popular, with local actress Chen Liping fronting their marketing campaign in December 2001. However, cases of consumers experiencing adverse health side effects came to light in 2002.[2] Selvarani Raja, a Slim 10 user had died from liver failure caused by the pills. Local actress Andrea De Cruz underwent a liver transplant after her liver had failed as well. Both cases led to lawsuits.[3]
Details of the incident
The Slim 10 pills were initially tested and approved for sale in November 2001 by the Health Sciences Authority (HSA).[4] The pills were manufactured in Guangdong, China, by Yuzhitang Health Products. The main importer of the pills was Health Biz Private Limited, and the main retail distributor of the pills was TV Media.
According to a report by local news outlet TODAY, TV Media had sold “over 16,000 bottles of the drug to companies such as NTUC Healthcare Pharmacy, Watson’s and Guardian SEA” between 3 December 2001 and 20 April 2002.[5] Each bottle of Slim 10 retailed at S$149.90 and contained 120 pills.[6]
First reports and investigation
The first report of adverse drug reactions was in late March 2002, where the victim experienced acute hepatitis.[7] A month later in April, two others experienced hyperthyroidism while another experienced menstrual irregularity. The HSA subsequently decided to launch an investigation into the ingredient composition of the Slim 10 pills.
It was discovered that Nicotinamide and Fenfluramine were undeclared ingredients present in the pills. Fenfluramine is a controlled substance under the Poisons Act.[8] Anyone found guilty under the Poisons Act is liable to a fine not exceeding SGD$10,000 or imprisonment for a term not exceeding 2 years or both, upon conviction.[9] It was later revealed that the Slim 10 pills also contained 2 other prohibited substances, thyroxine and triiodothyronine.[10]
Product withdrawal
By 19 April 2002, all Slim 10 stocks were withdrawn from the market. An estimated 20,000 bottles of Slim 10 had already been sold in Singapore.[11] By May 2002, a total of 13 reports related to adverse drug reactions were received by the HSA. Doctors also confirmed the adverse side effects of consuming Slim 10 pills included hyperthyroidism and liver toxicity.[12]
Andrea De Cruz’s hospitalisation
In the months of April and May 2002, local actress Andrea De Cruz had been admitted and transferred to 3 different hospitals in Singapore for treatment. The details provided in the following paragraphs were retrieved from an official court report dated 3 October 2003.[13]
Diagnosis (Changi General & Mount Alvernia Hospital)
On 15 April 2002, Andrea De Cruz went to see a doctor at Changi General Hospital following comments from her sister that her eyes looked very yellow and jaundiced.[14] At the hospital, the doctor confirmed that she was suffering from an inflamed liver, and had to be admitted. She was subsequently transferred to Mount Alvernia Hospital.
Despite various medical tests over 2-weeks, the hospital staff could not identify the source of her ailment.[15] During a liver biopsy, Andrea had allegedly started to convulse uncontrollably. In the end, the results of the liver biopsy were inconclusive.
Liver dialysis (National University Hospital)
On 3 May 2002, a doctor from the NUH liver team visited Andrea and prescribed her a few drugs which she vomited out soon after consuming. The next day, Andrea was transferred to NUH. Within 24 hours, she exhibited signs of brain damage. She was then sent to the Intensive Care Unit, where she immediately underwent liver dialysis.[16]
It was reported that Andrea De Cruz required a liver transplant. However, the policy then on liver transplants only permitted blood-related relatives to be donors, and all her relatives were found to be unsuitable. In the end, her then-fiance, Pierre Png, was found to be a suitable and willing donor. Andrea De Cruz’s family managed to gain the approval from the Ministry of Health to allow him to donate part of his liver to her, despite them being unrelated by blood.[17]
Liver transplant (Gleneagles Hospital)
On 7 May 2002, Andrea De Cruz was transferred to Gleneagles Hospital, as the NUH liver team did not have the experience of conducting a living donor transplant before.[18] At Gleneagles Hospital, Andrea De Cruz was further diagnosed with a thyroid problem and given medication to suppress it. The total time taken for the liver transplant surgery was 12 hours.
The surgery was a success and Andrea was discharged about 2 weeks later. She held a press conference on the same day of her discharge.
Lawsuit against Slim 10
Andrea De Cruz did not initially admit to taking the Slim 10 pills.[19] Her manager and brother-in-law had allegedly advised her against doing so. Firstly, Andrea's manager was worried about the adverse repercussions Chen Li Ping would face as she was the face of the Slim 10 pills. Andrea's brother-in-law argued that she should not be seen blaming Slim 10 unless it was confirmed that Slim 10 was the main cause of her liver failure.
In June 2002, Andrea De Cruz filed a negligence suit against Slim 10, Yuzhitang Health Products, Health Biz Pte Ltd, Semon Liu, TV Media and fellow local actor Rayson Tan. Rayson Tan was the director of TV Media and the husband of Chen Li Ping. He had allegedly sold the Slim 10 pills to Andrea De Cruz personally.[20]
The court concluded the case on 3 October 2003, ruling in favour of Andrea De Cruz. Health Biz Pte Ltd and TV Media were ordered to pay a total of S$1,830,000 for medical compensation, suffering, loss of income and the legal costs incurred. The two firms submitted a joint appeal, and the court eventually revised the compensation amount to S$1,630,000.[21]
Andrea De Cruz’s claims against Rayson Tan were dismissed by the court, and she was ordered to pay for the legal costs he had incurred to defend himself from her claims.[22]
Selvarani Raja’s death
On 9 May 2002, Selvarani Raja, who was a 43-year old logistics executive, was hospitalised in NUH for jaundice.[23] She had consumed the Slim 10 pills for a period of 3 months, from January 2002 to March 2002. This led to her having liver toxicity, which eventually led to liver failure. Although many were willing to be liver donors, she was unable to find a suitable liver donor.
She passed away on 1 June 2002. According to the state coroner’s report in March 2003, her liver had indeed failed due to her consumption of the Slim 10 pills.[24]
Lawsuit against Slim 10
In February 2005 Sharlin Raja, the sister of Selvarani Raja filed a lawsuit against TV Media and NTUC Healthcare, who retailed the Slim 10 pills after TV Media distributed it to them.[25] The suit claimed damages for psychiatric injuries that their family experienced due to Selvarani Raja’s death, the fact that her death was wrongful, as well as the income their family lost due to her death. In the end, all parties settled the case out of court.[26]
Charges pressed against Health Biz Pte Ltd and TV Media
Health Biz Pte Ltd and director Semon Liu
According to a press release by HSA on 26 September 2006, Health Biz Pte Ltd and its director Semon Liu were charged with the following for violating the Medicines Act:[27]
- 8 charges for selling Slim 10 without the necessary declaration that the product was free from poisons and any synthetic active substances, as required under Regulation 3(2)(d)(i) of the Medicines (Licensing, Standard Provisions and Fees) Regulations.
- 1 charge for failing to keep records of the importation of Slim 10, as required under standard provision 5(1) in the 2nd Schedule to the Medicines (Licensing, Standard Provisions and Fees) Regulations
Health Biz Pte Ltd pleaded guilty to all the charges. On 26 September 2002, the court imposed an S$45,000 fine on Health Biz Ptd Ltd for the above offences and revoked their wholesale dealer and import licenses for Chinese proprietary medicines (CPM).
It was also revealed in court that the Slim 10 pills were “imported into Singapore from China through air-parcel as unlabelled capsules in aluminium foil or plastic bags. They were unaccompanied by any documentation as to their identity or source. The parcels were either unmarked, or marked as ‘Gifts’, and the contents were not declared to the Customs authorities in Singapore”.[28] This was proof of the irresponsible manner as to which the company managed the import of the Slim 10 pills, and was deemed as “aggravating conduct” by HSA.
TV Media
TV Media was fined S$64,000 for 30 counts of selling Slim 10 without a wholesale licence. They also sold the Slim 10 pills even after the product had been banned and recalled by HSA. By June 2003, the company shut down the last 3 retail outlets which were located in Centrepoint, Bugis Junction and Shaw Towers.[29] TV Media also refunded all customers who had purchased Slim 10 pills from them.[30]
References / Citations
- ↑ “Slimming agent may affect heart”. The Straits Times. May 1, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020501-1.2.45.20
- ↑ Liang, H.T. “13 down from hepatitis, thyroid problem after taking Slim 10”. The Straits Times. May 7, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020507-1.2.25.13
- ↑ The Must Share News Team. “The Slim 10 Saga: Confusing Verdicts In Singapore Explained — Part 3”. MustShareNews. August 10, 2017. Accessed on 15 April 2019. Retrieved from: https://mustsharenews.com/slim-10-saga/
- ↑ “China bans liver risk diet pill”. BBC News. July 13, 2002. Accessed on 15 April 2019. Retrieved from: http://news.bbc.co.uk/2/hi/asia-pacific/2126691.stm
- ↑ Frances, Joy. “TV Media's Slim 10 troubles”. TODAY. July 17, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/today20020717-1.2.11.2
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “ADVERSE DRUG REACTION NEWS”. Health Sciences Authority. August, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Reaction_News/2002/ADR_News_Aug2002_Vol4No2.pdf
- ↑ “HSA DETECTS FENFLURAMINE, A CONTROLLED SUBSTANCE, IN THE CHINESE PROPRIETARY MEDICINE “SLIM 10””. Health Sciences Authority. April 30, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/News_and_Events/Press_Releases/2002/PRelease-HSA%20Detects%20Fenfluramine%20in%20Slim10-30April02.pdf
- ↑ “HSA DETECTS FENFLURAMINE, A CONTROLLED SUBSTANCE, IN THE CHINESE PROPRIETARY MEDICINE “SLIM 10””. Health Sciences Authority. April 30, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/News_and_Events/Press_Releases/2002/PRelease-HSA%20Detects%20Fenfluramine%20in%20Slim10-30April02.pdf
- ↑ Wong, S.M. “13 down from hepatitis, thyroid problem after taking Slim 10”. The Straits Times. June 19, 2002. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20020619.2.29.10
- ↑ “ADVERSE DRUG REACTION NEWS”. Health Sciences Authority. August, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Reaction_News/2002/ADR_News_Aug2002_Vol4No2.pdf
- ↑ Chong, Elena. “Slim 10 Negligence suit starts amid buzz”. The Straits Times. June 23, 2003. Accessed on 15 April 2019. Retrieved from: http://eresources.nlb.gov.sg/newspapers/Digitised/Article/straitstimes20030623.2.26.9
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ Khalik, S. Liang, H.T. “Dying Andrea’s boyfriend donates liver”. The Straits Times. May 8, 2002. Retrieved from NewspaperSG.
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ “De Cruz Andrea Heidi v Guangzhou Yuzhitang Health Products Co Ltd and Others [2003] SGHC 229”. Singapore Law Watch. October 3, 2003. Accessed on 15 April 2019. Retrieved from: https://www.singaporelawwatch.sg/Portals/0/Docs/Judgments/%5B2003%5D%20SGHC%20229.pdf
- ↑ Vijayan, K.C. “Court rules in favour of Andrea but damages cut.”. The Straits Times. July 9, 2004. Retrieved from NewspaperSG.
- ↑ Chan, C. “Pay up your share of damages costs.”. The New Paper. September 3, 2006. Retrieved from NewspaperSG.
- ↑ Liang, H.T. “Slim 10: Another woman needs liver transplant”. The Straits Times. May 28, 2002. Retrieved from NewspaperSG.
- ↑ Chong, Elena. “Slim 10 Negligence suit starts amid buzz”. The Straits Times. June 23, 2003. Retrieved from: NewspaperSG.
- ↑ Singh, K. “Slim 10 victim’s sister files lawsuit.”. The Straits Times. February 23, 2005. Retrieved from NewspaperSG.
- ↑ Vijayan, K.C. “Victim's sis and 2 parties settle suit”. The Straits Times. June 1, 2005. Retrieved from NewspaperSG.
- ↑ “CONVICTION OF HEALTH BIZ PTE LTD AND REVOCATION OF ITS IMPORT AND WHOLESALE DEALER’S LICENCES”. Health Sciences Authority. September 26, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/News_and_Events/Press_Releases/2002/PressRelease-ConvictionOfHealthBizRevocationOfLicences-26Sep02-website.pdf
- ↑ “CONVICTION OF HEALTH BIZ PTE LTD AND REVOCATION OF ITS IMPORT AND WHOLESALE DEALER’S LICENCES”. Health Sciences Authority. September 26, 2002. Accessed on 15 April 2019. Retrieved from: https://www.hsa.gov.sg/content/dam/HSA/News_and_Events/Press_Releases/2002/PressRelease-ConvictionOfHealthBizRevocationOfLicences-26Sep02-website.pdf
- ↑ Chong, Elena. “Slim 10 Negligence suit starts amid buzz”. The Straits Times. June 23, 2003. Retrieved from NewspaperSG.
- ↑ “Refunds for slimming pills.”.The Straits Times. May 10, 2002. Retrieved from NewspaperSG.